Novo Compliance ensuring our clients achieve their achieve their regulatory commitments to Patients, Healthcare Providers and Global Regulatory Authorities.
Services and solutions throughout the lifecycle of product development
Mitigating Sponsor’s Global Regulatory Challenges
Streamlining Sponsor’s Regulatory Compliance implementing Novo’s Regulatory Strategic ARC®
Spearheading Organizational Efficiencies throughout the Product development Life Cycle
Mitigating Sponsor’s Global Regulatory Challenges
Streamlining Sponsor’s Regulatory Compliance implementing Novo’s Regulatory Strategic ARC®
Spearheading Organizational Efficiencies throughout the Product development Life Cycle
Mitigating Sponsor’s Global Regulatory Challenges
Streamlining Sponsor’s Regulatory Compliance implementing Novo’s Regulatory Strategic ARC®
Spearheading Organizational Efficiencies throughout the Product development Life Cycle
Mitigating Sponsor’s Global Regulatory Challenges
Streamlining Sponsor’s Regulatory Compliance implementing Novo’s Regulatory Strategic ARC®
Spearheading Organizational Efficiencies throughout the Product development Life Cycle
Explore Our Core Services
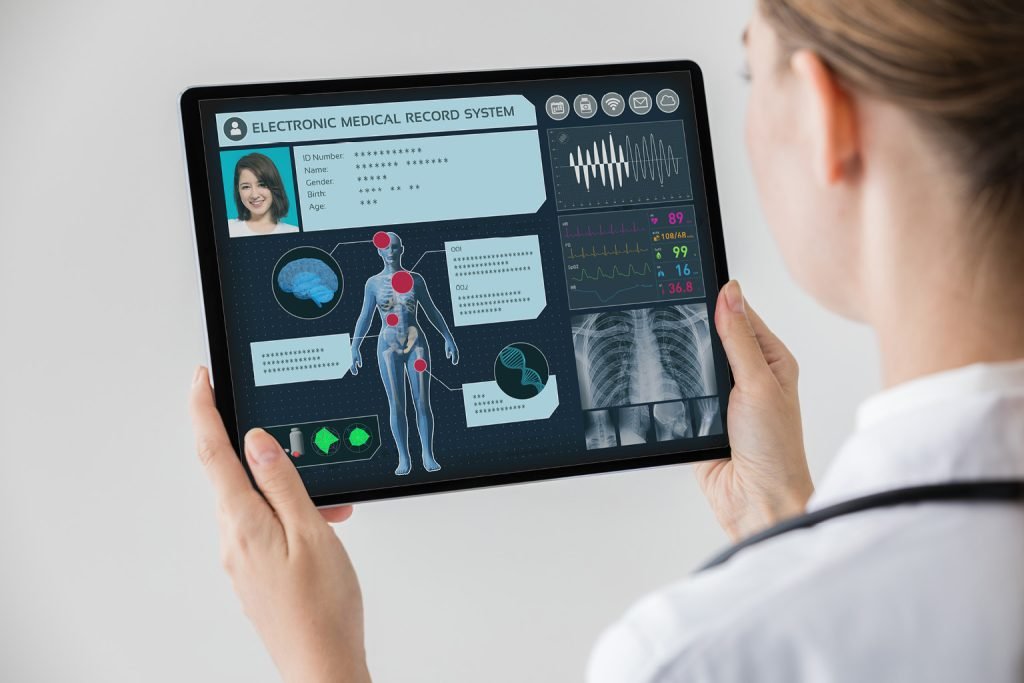
Regulatory Compliance (RC) in the life sciences sector, spanning pharmaceuticals, biotechnology, and medical devices.

Regulatory Intelligence (RI) is the systematic process of gathering, analyzing, and applying regulatory information to guide strategic decisions.

Regulatory Sciences is the discipline that underpins the development, evaluation, and oversight of medical products through scientific methods.

Regulatory Compliance (RC) in the life sciences sector, spanning pharmaceuticals, biotechnology, and medical devices.

The importance of regulatory compliance and a systematic risk management approach in early stages of the product lifecycle can’t be overstated.

Novo Compliance’s Virtual Team Model delivers timely, targeted regulatory advice, guidance, and support combining both the flexibility of immediately available.

Design, execution, and oversight of clinical trials to generate safety and efficacy data. Designing Phase clinical trial protocols. Overseeing patient safety and trial conduct.

Regulatory Intelligence (RI) is the systematic process of gathering, analyzing, and applying regulatory information to guide strategic decisions.
“Accelerating global therapeutic innovation by guiding clients, partners, and healthcare providers in architecting resilient, role-specific, and harmonized regulatory strategies through adaptive life science frameworks.”
Empowering our clients and partners from early Research & Development pre-clinical phase developing life changing breakthroughs in therapeutic advancements benefiting unmet medical needs across the global.
Novo Compliance’s team members are availabile supporting our clients with industry leading expertise spanning multiple therapeutics domains and specialties. Our virtual and embedded resources offer unique, timely and comprehensive local and remote expertise, addressing urgent and mission critical regulatory and quality challenges…in the moment!
Systems Architecture Emphasis
Vision Statement:
Novo Compliance envisions a globally harmonized life sciences ecosystem where regulatory intelligence, quality systems, and innovation converge through adaptive, ontology-driven architectures. By institutionalizing our proprietary Regulatory Compliance ARC® and modular audit simulation frameworks, we empower organizations to anticipate change, accelerate market access, and sustain compliance across the full product lifecycle from early development to post-market stewardship.
Mission Statement:
Novo Compliance envisions a globally harmonized life sciences ecosystem where regulatory intelligence, quality systems, and innovation converge through adaptive, ontology-driven architectures. By institutionalizing our proprietary Regulatory Compliance ARC® and modular audit simulation frameworks, we empower organizations to anticipate change, accelerate market access, and sustain compliance across the full product lifecycle from early development to post-market stewardship.

Digital Transformations
Our organization provides bespoke consulting services supporting our clients’ Digital Transformations processes in Pharmaceuticals, Biologics and Medical Devices from development, through commercialization, deployment, maintenance and global transformative integration of advanced technologies. Our global support includes AI&ML, Cloud platforms, and Customized Automation from R&D, in clinical trial management, in product manufacturing and commercialization, leading to more inclusive patient engagement, by accelerating innovations, scaling operational efficiencies and improving outcomes for patients, healthcare practitioners and with sponsors, improving standard of care and addressing challenges with unmet medical needs.
We add value by focusing on understanding your business and applying our experience and implementation approach.
Our Focus
We prioritize strategic clarity, sustainable growth, and measurable impact, ensuring businesses stay ahead in a dynamic market.
Our Approach
Combining data-driven insights with tailored solutions, we create actionable strategies that drive real business results.
Our Experience
Years of hands-on consulting have helped organizations navigate complexity, unlock growth, and achieve lasting transformation.
Leadership Guidance
Thought Leadership in Life Sciences
Novo Compliance, LLC© senior leadership team have deep expertise working with our clients and sponsors in collaborations with executive & senior management and functional leads as thought leaders and peers strategically developing dynamic forward-thinking principles, key elements including life cycle ecosystem innovations, data-driven methodologies and strategic insights; bridging technical complexities with audience access, work-flows and KPIs;
Resource Library in Life Sciences
Novo Compliance’s Resource Library provides information for our clients related to global regulatory frameworks governing pharmaceuticals, biologics, and medical devices, on various pathways required by major authorities including the FDA, EMA, MHRA, PMDA, TGA and WHO, providing harmonization with regulations, guidance’s common specification and consensus standards advancing marketing access.
Customized Trainings
Our Trainings
- QMSRs training
- cGxP training
- MDR training
- IVDR training
- Premarket Notification (PMN 510K)
- Premarket Approval (PMA)
- Medical Device Classification Request (513g)
- Combination Products 21CFR Part 4
Careers
- Senior Data Scientist AIML & Computational Sciences
- Senior Medical Writer
- Director of Training
- Clinical Affairs Associate Director
- Clinical Affairs Director
- Director R&D
Technical Resource – Project - Management Tools JIRA & Smartsheet’s
We believe our people deserve an environment where they can excel and thrive…supported, respected, and empowered, growing their careers through contributing individually, collaborating on teams, and participating in key roles with client’s, sponsors, and healthcare provider’s, shaping meaningful, impactful, and beneficial patient-focus outcomes.
What Our Clients Say
Change Starts With a Conversation
Have a Challenge or an Idea?
Call Us At:
123 123 123
Visit Us At:
Los Angeles CA, London UK, Novo Compliance©, LLC
Contact Form
Let's discuss your next project
Partner with us for strategic solutions that drive success.




